In humans, oral fluids containing both pathogens and antibodies are used to test for a variety of infections, e.g., HIV, measles, etc. In pigs, research has shown that oral fluids can be used to detect PRRSV, PCV2, SIV, and M. hyopneumoniae infections.
Oral fluid testing available at ISU VDL
- Mycoplasma hyopneumoniae PCR
- PRRS ELISA X3 - see more info
- PRRS PCR
- PRRS Quantitation
- SIV PCR
- SIV VI
Prognostic Profiling of Swine Populations Using Oral Fluid Samples
John Prickett, John Johnson, K-J Yoon, Lorraine Hoffman, Jeff Zimmerman
Veterinary Diagnostic Laboratory, Iowa State University, Ames, IA
What is oral fluid prognostic profiling?
Prognostic profiling is a process for monitoring the circulation of pathogens in swine populations. The process is "prognostic" because the goal is to forecast the health and productivity of the population in the immediate future. The process uses oral fluids because they are quick, easy, and inexpensive to collect. Prognostic profiling is not a diagnostic procedure. Pigs showing clinical signs should be evaluated using conventional diagnostic methods.
What is "oral fluid"?
Oral fluid is the liquid present in the oral cavity. Oral fluid is a mixture of saliva and "oral mucosal transudate". Saliva is produced by the salivary glands. Oral mucosal transudate enters the mouth by crossing the buccal mucosa from the capillaries. Oral fluids contain both pathogens and antibodies. In humans, oral fluids are used to test for a variety of infections, e.g., HIV, measles, etc. In pigs, research has shown that oral fluids can be used to detect PRRSV, PCV2, SIV, and M. hyopneumoniaeinfections.1,2,3
How to collect oral fluid samples
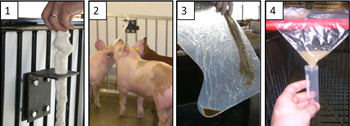
View a PDF prepared by the Center for Food Security and Public Health.
Fig 1. Suspend a length of cotton rope in a location accessible to the pigs. Ropes should be placed in a clean area of the pen and not in close proximity to water or feed. Cotton rope is recommended because it is highly absorbent. Use 1/2" (1.3 cm) rope for nursery pigs; 5/8" (1.6 cm) rope for grow-finish pigs. The figure (above) shows a bracket with a 1" (2.5 cm) hole in the horizontal plate to hold the rope. A knot in the top of the rope secures it in place during collection.
Fig 2. Hang rope shoulder high to the pigs (hang the rope, then cut to length). The pigs deposit oral fluids as they chew the rope. In active pens, 20-30 minutes is sufficient sampling time.
Fig 3. Extract oral fluids from the rope. Insert the bottom (wet) end of the rope into a clean plastic bag or single-use plastic boot. Squeeze the rope so that the fluid accumulates in one corner.
Fig 4. Cut a corner of the plastic and drain the contents into a tube (Falcon 2054 or equivalent). A 4 ml sample is ideal for testing. If samples are clean, no further processing is necessary. If samples contain particulates, centrifuge for 10 minutes and then pour into a clean tube.
Handling oral fluid samples
- Freeze samples promptly to optimize quality. Oral fluid samples for same day submission may be chilled and submitted on wet ice. Maintain the cold chain to preserve sample integrity.
- Prepare and handle samples for shipment to the testing laboratory as is required for serum samples, i.e., pack samples with ice packs and absorbent material (to capture spills) in an insulated leak-proof transport container lined with a plastic bag.
Sampling strategy
- Prognostic profiling relies on testing oral fluid samples at approximately 2-week intervals for specific pathogens of interest. To optimize detection of pathogens, sample pens spaced throughout the facility. Sampling the same pens repeatedly over time provides longitudinal data on the population.
- Sample size calculations for specific pathogens have not been established. Field studies showed that sampling 6 pens in 1,100 head wean-finish barns at 2-week intervals detected circulation of PRRSV, PCV2, and SIV.3
Interpretation of lab results
- PCR-positive results reflect active circulation of pathogens in the population. Caution: to avoid false negative results, PCR procedures optimized for serum must be validated for oral fluid samples. Check with the laboratory prior to submission.
- A PRRSV ELISA for the detection of antibodies in oral fluids is available through the ISU VDL.
Trouble-shooting oral fluid collection
- Do not pool oral fluid samples.
- Cotton rope is usually unavailable at local stores, but can be purchased through the Internet. Other materials, especially synthetic materials, do not have the absorbency of cotton.
- Pigs are occasionally reluctant to approach the rope for the first collection. Reluctant pigs can be trained by throwing a length of rope in the pen for them to play with or by flavoring practice ropes with sugar solution. Once accustomed to the rope, pigs look forward to the next collection.
- Pigs are more active in the morning. Afternoon collections may require more time.
- Dirty samples are often an indication that the rope was too long. Hang rope at pig shoulder height.
- Pigs are attracted to the rope because it is something new. Remove all ropes after each sampling to keep their interest in future collections. Discard rope after use; never re-use.
References cited
1. Prickett J, Simer R, Christopher-Hennings J, et al. 2008a. Detection of porcine reproductive and respiratory syndrome virus infection in porcine oral fluid samples: A longitudinal study under experimental conditions. J Vet Diagn Invest 20:156-163.
2. Prickett J, Simer R, Yoon K-J, et al. 2008b. Oral-fluid samples for surveillance of commercial growing pigs for porcine reproductive and respiratory syndrome virus and porcine circovirus type 2 infections. J Swine Health Prod 16(2):86-91.
3. Hoffman P, Prickett J, Zimmerman J, et al. 2008. Implementation and validation of swine oral fluid collection in a commercial system. Proceedings of the 38th Annual Meeting of the American Association of Swine Veterinarians. San Diego, California, pp. 301-302.
